Abstract
INTRODUCTION The introduction of post-transplant cyclophosphamide (PTCY) for GVHD prevention can be considered a milestone in haploidentical hematopoietic cell transplantation (haplo-HCT). And secondary to its efficacy, the use of haplo-HCT with peripheral blood stem cell (PBSC) grafts has significantly increased in last decade. However the optimal immunosuppressive agents to combine with PTCY using peripheral blood stem cell (PBSC) sources is still a matter of debate, as in a retrospective analysis conducted by CIBMTR using PBSC grafts and PTCY-based GVHD prophylaxis in the haplo-HCT setting, was associated with higher rates of acute and chronic GVHD than with using bone marrow stem cell grafts.
PTCY-based prophylaxis is considered a standard of care when haploidentical donors are selected. PTCY combined with anti-thymocyte globulin and cyclosporine (PTCY-ATG-CsA) became our institutional GVHD prophylaxis for peripheral blood (PB) haplo-HCT in 2015. The present analysis aims to share updated information about our experience using ATG-PTCY-CsA for GVHD prevention in PB haplo-HCT.
METHODS Between October 2015 to December 2021, 157 consecutive adults underwent haplo-HCT at our institution and were included in the study. The GVHD prophylaxis was composed of rabbit-ATG (Thymoglobulin; Genzyme-Sanofi, Lyon, France), PTCy (50 mg/kg/day on days +3 to +4), and CsA (started on day +5 and titrated to therapeutic levels ranging between 200 and 400 ng/ml). One hundred twenty (76.4%) adults received a total dose of 4.5mg/kg of ATG (0.5 mg/kg on day -3, 2 mg/kg on day -2 and 2 mg/kg on day-1), and 37 (23.5%) received 2 mg/kg (0.5 mg/kg on day -2 and 1.5 mg/kg on day -1). T-cell replete PBSC grafts were infused on day 0 in all cases. Data was collected retrospectively and updated in March 2022. To perform regression analyses, post-transplant follow-up was censored at 2 years.
RESULTS Of the 157 patients, the median age was 57 years (range: 43-66), and 67 (44%) were 60 years or older. Sixty-four (44%) patients were females. Acute myeloid leukemia was the most prevalent baseline diagnosis (n=79, 50.3%), and 38 (24.2%) patients received myeloablative conditioning (MAC) regimens. Forty-six (30%) adults had a KPS ≤80%, 46 (30%) had an HCT-CI score >3, and 34 (23.4%) had a high or very-high disease risk index (DRI). Fresh products were infused in 90 (57.32%) cases.
A total of 152 patients achieved engraftment. The median time for neutrophil and platelet engraftment were 17 (IQR 19-15) and 22 days (IQR: 12-28). The cumulative incidence function (CIF) of bloodstream infection at day +28 was 51.6%. The day +180 CIF of CMV and EBV reactivations were 62.5% and 65.4%, respectively, and 5 (3.2%) patients had post-transplant lymphoproliferative disorder.
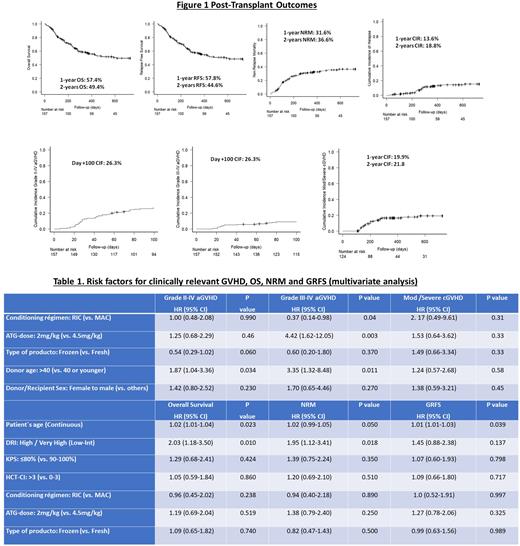
The CIFs of grade II-IV and grade III-IV acute GVHD estimated at day +100 were 26.3% and 9.5%, and the CIF of moderate/severe cGVHD at 1 year was 19.9%. As reported in Table 1, the use of MAC regimens (HR 2.70), the administration of 2 mg/kg of ATG (HR 4.42, P=0.003) and the selection of donors older than 40 years (HR 3.35, P=0.011) increased the risk for grade III-IV aGVHD. The infusion of frozen products was not found to be predictor for GVHD. Nevertheless, a non-significant trend to lower grade II-IV aGVHD was documented in patients receiving frozen PBSC grafts (HR 0.54, P=0.060).
The median follow-up in survivors was 32 months. Overall, 73 (46.5%) patients died, and 28 (17.3%) relapsed. The leading causes of death were infection and relapse. The median of days to discontinuation of the immunosuppression in survivors was 83 days (IQR: 69-119). As shown in Figure 1, the estimated 2-year OS, RFS, NRM, CIR, and GRFS were respectively: 49.4%, 44.6%, 36.6%, 18.8%, and 35.7%. The multivariate regression analysis reported n Table 1 showed that older patients and those with high and very-high DRI were at higher risk for worse OS and higher NRM.
CONCLUSION Dual T-cell depletion using PTCy and ATG (total dose of 4.5mg/kg) combined with CsA is a safe drug combination for PB haplo-HCT. The use of this prophylaxis provided an effective GVHD prevention with acceptable relapse rates. Moreover, the selection of younger donors, and the use of a total dose of 4.5mg/kg of ATG increased the effectiveness of GVHD prevention.
Further research is needed to decrease rates of NRM for patients undergoing haplo-HCT using this prophylaxis.
Disclosures
Law:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Educational: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Atara: Research Funding; Incyte Corporation: Research Funding; Sierra: Research Funding. Kim:Pfizer: Consultancy, Honoraria, Research Funding; Merck: Consultancy; Paladin: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal